SciGen Teacher Dashboard
Unit M3
Close-up Look at Change
Concentrating on Pink Lemonade
Categories of Chemicals and Mixtures
Condensation and Evaporation
Describing Physical and Chemical Change
The Three Little Chemists and the Big Bad Wolf
 Conversation: Describing Physical and Chemical Change
Conversation: Describing Physical and Chemical Change
Duration: Approximately 45 minutes
This activity focuses on how to discern and describe the difference between physical and chemical change. First the students consider the difference between a bending nail and a rusting nail. They go on to look again at phase change. Burning is offered as an example of chemical change, and other common indicators of chemical change are mentioned. Finally, students try their hands at classifying familiar examples of change into physical and chemical.
LEARNING OBJECTIVE
Students will compare physical and chemical change.
Students will consider whether phase change is a physical or chemical change.
Students will classify familiar changes as physical or chemical change.
Teacher Tune-ups
Teaching Notes
ACTIVITY OVERVIEW
- Physical and chemical change: notice the difference (15 minutes)
- Phase changes (5 minutes)
- What about burning? (10 minutes)
- Categorize change (15 minutes)
Physical and chemical change: notice the difference (15 minutes)
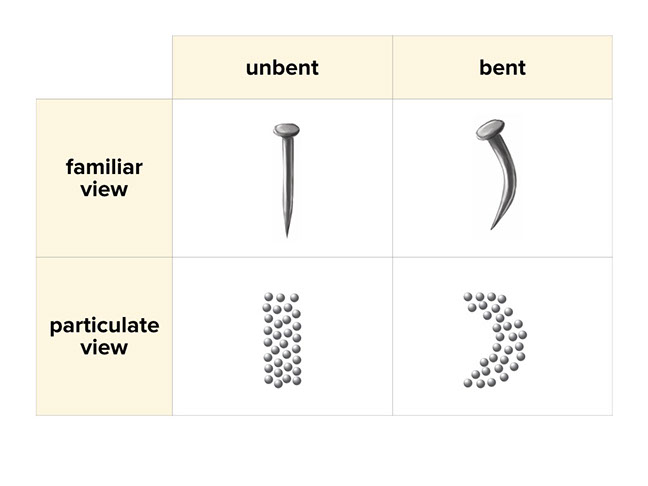
The bending nail is an example of a physical change. Students can see that there is no change in the particles (the smallest parts) of the nail as a result of bending. Their position changes, but not their chemical identity: they are still all iron atoms.
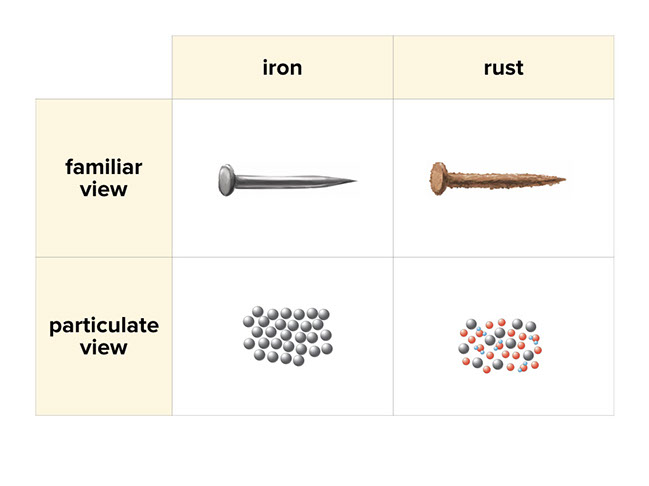
Ask students what they think happens when a nail is exposed to moisture. This is no longer a physical change but a chemical change. The second slide explains a chemical reaction.
Ask students to contrast the types of change.
:
- “The straight nail is the same color and texture as the bent nail; whereas the nails on this page are different colors and textures.”
- “The particles of the straight nail and the bent nail are the same; however, the particles of the rusty nail are different than those of the iron nail.”
Explain to students that there has been what we call a chemical reaction–the iron from the original nail, oxygen in the air, and water are like the “ingredients” of rust. However, instead of ingredients, we use the more scientific vocabulary “reactants.”
Phases changes (5 minutes)
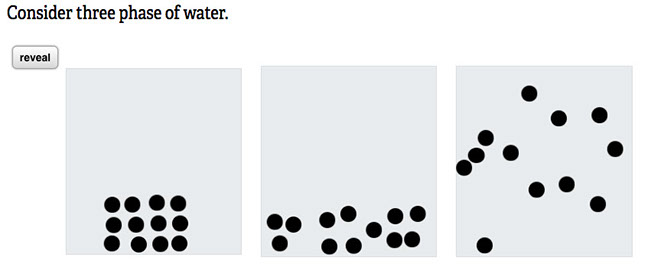

Show the animation of solid, liquid and gas at the particulate level and ask students to identify them and to discuss their properties.
Show second panel by clicking "reveal."
Ask students to consider if there is physical or chemical change when water is heated and cooled in this manner.
:
physical
What about burning? (10 minutes)
:
What about burning? Do you think that is a physical or chemical change?
:
- In this case, there was a chemical change: the wood reacted with oxygen in the air and literally went up in smoke, changing from cellulose to soot, ash, water, and carbon dioxide. Without knowing details of reactants and products, students may recognize that burning changes the chemical identity of substances, not just their phase or arrangement.
Mention to students that light and heat are often sign of chemical change. Common indicators of chemical change are:
- Light
- Change in temperature
- Formation of gas (like baking soda and vinegar—but this is different from carbon dioxide coming out of solution in soda, which is just a phase change, not a chemical reaction)
- Formation of precipitate, a solid product of gases or liquids reacting. Not to be confused with solids settling, like sediment in dirty water.
- Change in odor
- Change in color
- Change in pH
Categorize change (15 minutes)
Do this section as a class discussion, in groups, or as an individual assessment.
:
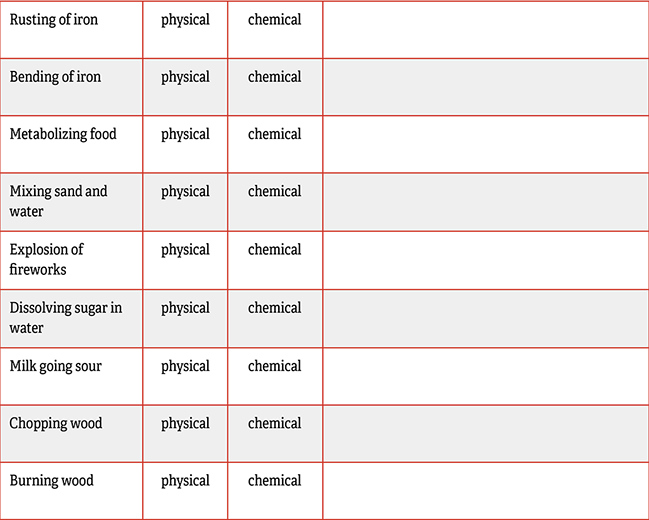
- Rusting of iron: Chemical. Explanation: When iron rusts, 3 oxygen atoms combine with every 2 iron atoms.
- Bending of iron: Physical. Explanation: Iron atoms don’t combine with different elements when iron bends.
- Metabolizing food: Chemical. Explanation: Molecules in food get broken down. For example, atoms from sugar get rearranged into carbon dioxide and water.
- Mixing sand and water: Physical. The water is still water and the sand is still sand. So the chemicals are unchanged.
- Explosion of fireworks: Chemical. Fire always involves a chemical reaction. Light and heat are often signs of chemical change.
- Dissolving sugar in water: Physical. When sugar dissolves, the sugar molecules separate from each other, but they are still sugar molecules.
- Milk going sour: Chemical. This example is like when we metabolize food, except here it’s bacteria metabolizing the milk.
- Chopping wood: Physical. Chopping wood just breaks the wood fibers apart. It doesn’t change the molecules in the wood.
- Burning wood: Chemical. The molecules in wood are broken apart when wood burns. A lot of their atoms are reorganized into carbon dioxide molecules, for example.
BETA Version - Please send comments and corrections to info@serpinstitute.org