SciGen Teacher Dashboard
Unit M3
Close-up Look at Change
Concentrating on Pink Lemonade
Categories of Chemicals and Mixtures
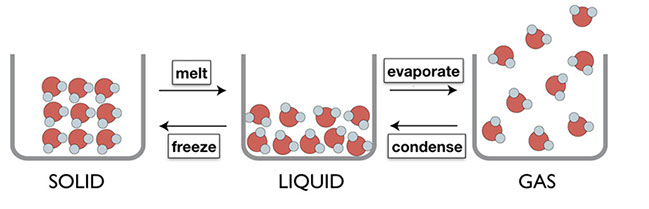
Condensation and Evaporation
Describing Physical and Chemical Change
The Three Little Chemists and the Big Bad Wolf
Interactive: Condensation and Evaporation
Duration: Approximately 45 minutes
Using the familiar examples of water droplets on the outside of an iced beverage and the phases of water (ice, liquid water, and water vapor), students explore the concepts of condensation and evaporation.
At the end of the activity, students are challenged to think through the water cycle and why rain is not salty.
LEARNING OBJECTIVE
Students will learn how condensation occurs.
Students will review the process of evaporation and consider the example of sea water.
Students will learns terms that describe phase changes.
Materials (one per student or group)
- optional: electronic device (such as computer, laptop, tablet) to use the interactive elements, if not looking at them as a class
Teacher Tune-ups
Teaching Notes
ACTIVITY OVERVIEW
- About condensation (15 minutes)
- About evaporation (15 minutes)
- Fresh water from the salty sea? (15 minutes)
About condensation (15 minutes)
:
Where does the water on the outside of cold glass come from?
Go through this interactive slide set as a class or assign to individuals or groups.
:
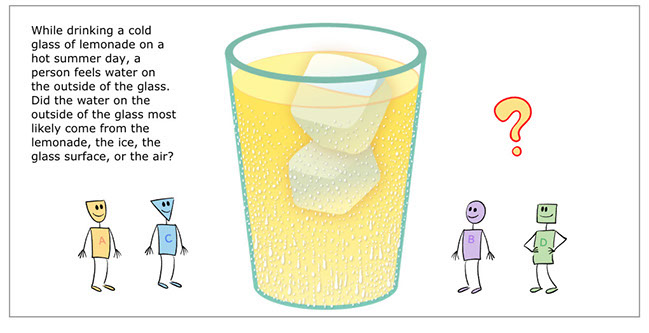
- While drinking a cold glass of lemonade on a hot summer day, a person feels water on the outside of the glass. Did the water on the outside of the glass most likely come from the lemonade, the ice, the glass surface, or the air?
:
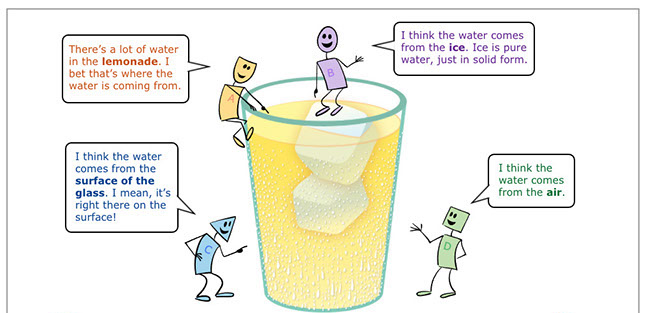
Students consider four different ideas.
- Idea A: The lemonade (wrong).
- Idea B: The ice (wrong).
- Idea C: The surface of the glass (wrong).
- Idea D: The air (right). The water that appears on the outside of the glass is from the air. Invisible water vapor in the air condenses into liquid water when it comes into contact with the cold surface of the glass.
:
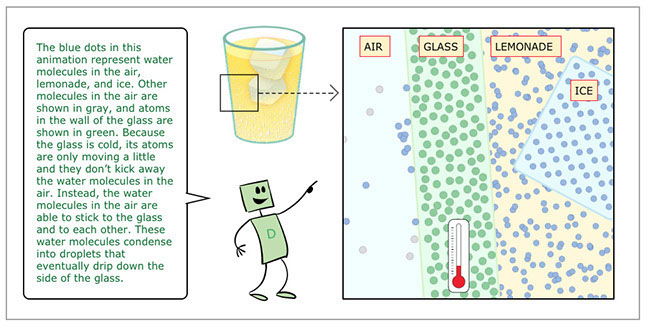
- The blue dots in this animation represent water molecules in the air, lemonade, and ice. Other molecules in the air are shown in gray, and atoms in the wall of the glass are shown in green. Because the glass is cold, its atoms are only moving a little and they don’t kick away the water molecules in the air. Instead, the water molecules in the air are able to stick to the glass and to each other. These water molecules condense into droplets that eventually drip down the side of the glass.
:
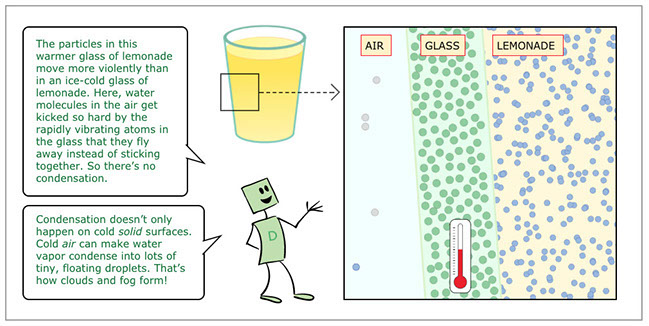
- The particles in this warmer glass of lemonade move more violently than in an ice-cold glass of lemonade. Here, water molecules in the air get kicked so hard by the rapidly vibrating atoms in the glass that they fly away instead of sticking together. So there’s no condensation. Condensation doesn’t only happen on cold solid surfaces. Cold air can make water vapor condense into lots of tiny, floating droplets. That’s how clouds and fog form!
About evaporation (15 minutes)
This section contains six slides related to evaporation for students to discuss.
:
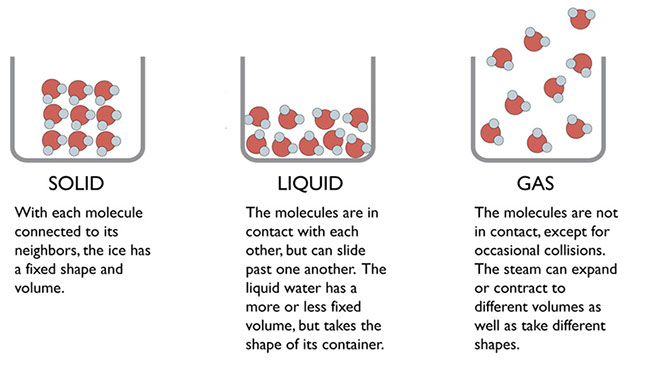
Building on the particulate views of mixtures from the previous section, this section starts by considering three phases of water. These three phases should be very familiar to students at this grade level.
Show the three particulate illustrations on this to the students. Ask them to consider what happens to water when it changes from one phase to another?
:
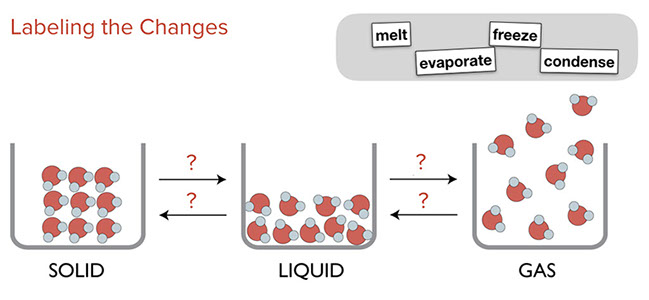
Ask students to think through how to label the arrows that represent the changes of water between phases.
- Click on "reveal" to show answers.
:
Read and discuss:
- For chemists, one thing that distinguishes a mixture from a compound is that the ingredients in a mixture can be taken apart by a physical process, while the ingredients of a compound can only be taken apart by a chemical reaction. You can get solid salt out of a saltwater mixture by evaporating the water molecules away, a physical process requiring no chemical reaction. This evaporation might be caused by, for example, sunlight heating the water, so that many of the water molecules get enough energy to escape the salt water and fly into the air.
:
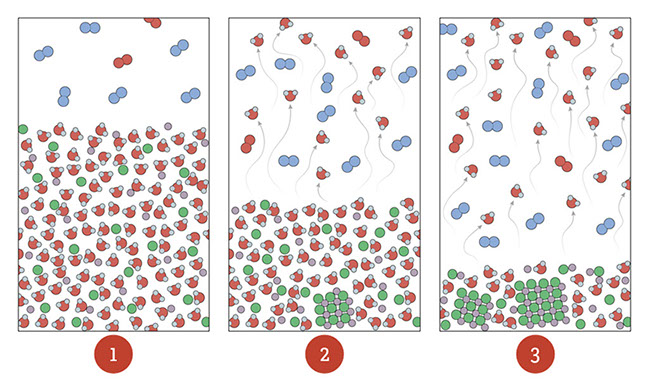
Tell students that the image #1 is a saltwater solution with air above. Ask students to consider what the images #2 and #3 show.
Paraphrase:
- When seawater evaporates, only the water molecules evaporate into the air. The sodium and chlorine atoms do not; they remain in the seawater. As more water evaporates, students should notice that the sodium and chlorine form crystals of sodium chloride, just as the powder from pink lemonade recrystallizes when the water evaporates in the Science Scene.
:
Show students the photograph of the salt pan worker. This is how we get sea salt to put in our food. Ask them if this is a natural process, a human-engineered process, or both.
Answer:
- Both


Fresh water from the salty sea?
 Water, water, everywhere,
Water, water, everywhere,
Nor any drop to drink.
— Samuel Taylor Coleridge,
The Rime of the Ancient Mariner

Ask students to read the lines from the Coleridge poem. Ask them if they’ve ever seen a movie or read a book where the character(s) were at sea and needed fresh water. What did the characters try to do to get freshwater?
(Some students may have read the novel or seen the movie Life of Pi, in which the protagonist captures fresh water from evaporated seawater using a floating “solar still.”)
:
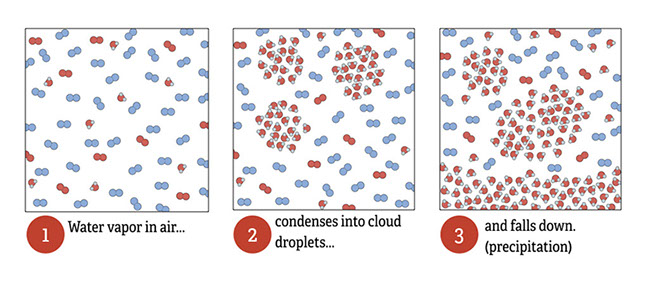
The three particulate illustrations shows air that has lots of water vapor in it. When the concentration of water vapor in the air gets high enough (relative to the temperature—it has to be cold enough), the water molecules clump together into liquid droplets. This transition from gas to liquid is called condensation. As more water condenses, droplets join together into larger drops and can fall as rain.
The water cycle diagram shows the processes of evaporation and condensation at work on a massive scale, in the water cycle.
Ask students to discuss the role that temperature plays in this process.
:
- In general, colder temperatures with high water vapor concentration in the air promote condensation. Higher temperatures and lower water vapor concentration work against concentration.
:
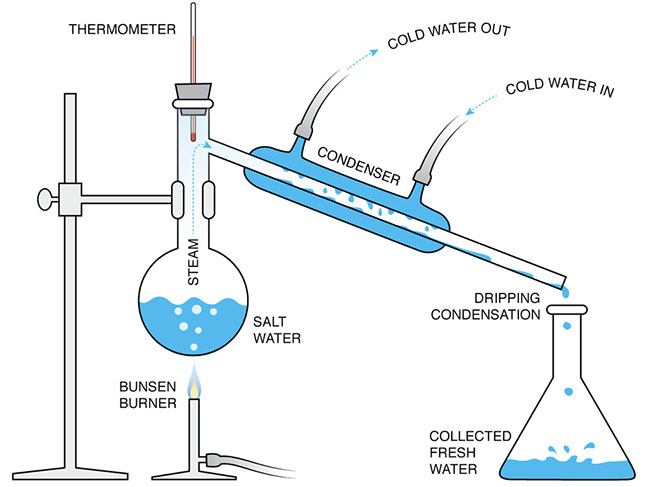
This illustration of the laboratory equipment shows how we can replicate the distillation process seen in the water cycle.
Underscore for students that the cold water circulating in and out of the condenser is only there to transfer heat away from the steam. None of that cold water passes into the tube where the steam is. All of the water that drips into the collection flask is condensed steam from the original salt water.
Ask students to label the equipment with the corresponding natural phenomena.
:
- Bunsen burner—sun
- salt water—the ocean
- condenser—cloud formation
- dripping condensation—rain
- collected fresh water—rivers and lakes
BETA Version - Please send comments and corrections to info@serpinstitute.org