SciGen Teacher Dashboard
Unit M3
Close-up Look at Change
Concentrating on Pink Lemonade
Categories of Chemicals and Mixtures
Condensation and Evaporation
Describing Physical and Chemical Change
The Three Little Chemists and the Big Bad Wolf
 Scene: Concentrating on Pink Lemonade
Scene: Concentrating on Pink Lemonade
Duration: Approximately 65 minutes
In this Science Scene, a dialogue to read aloud as a class, students take the roles of three friends making pink lemonade and considering what happens to the drink as they add more powder. They use precise vocabulary such as dissolve, dilute, and concentration when talking about the sweetness of the lemonade. They then consider different kinds of drink mixtures like lemonade vs. smoothies and discuss the distinction between homogeneous and heterogeneous mixtures. One of the friends is inspired to use the information on homogeneous mixtures (also called solutions) for a science poster. To reinforce concentration, students consider how blood alcohol concentration (BAC) is determined by the relationship between a person’s size and the amount of alcohol that they drink.
LEARNING OBJECTIVE
Students consider the difference between heterogeneous mixtures and homogeneous mixtures (solutions).
Students relate the concepts of dilute and concentrate to a solution.
Students discuss the effects of evaporation on the concentration of a solution.
Teacher Tips
- Think ahead about how you will assign the roles to read the script aloud as a class (or, less ideally, in small groups). Note that students often willingly take on roles regardless of the gender of the characters.
- While it is possible for the students to read the PDF of the script online, we suggest printing the script for the students so they can hold it in their hands and mark it as they read. Consider scaffolding the lesson for English language learners: you could let them preview the script before the lesson or read the dialogue silently once and ask questions to a partner.
- Prior to the reading the script, explain to students that they will encounter new vocabulary terms and ideas that may not be familiar. Reassure them that this lack of familiarity is okay. The reading is meant to introduce the ideas, not explain them completely.
- Review the focus words of the week. The focus word chart linked on the unit overview page should be used as a resource for students to review definitions and sample sentences.
Teacher Tune-ups
Teaching Notes
ACTIVITY OVERVIEW
- Set the context (10 minutes)
- Engage with the script (20 minutes)
- Considering Olivia's poster (20 minutes)
- About blood alcohol concentration (15 minutes)
Set the context (10 minutes)
Show video clip to students and ask what they believe is happening. Encourage various ideas, but let the students know that by the end of the activity they will have much more information about what is going on when powders dissolve in liquids.
Engage with the script (20 minutes)
Olivia: Where’s a pitcher? I’ll fill it with water.
Zena: Nope, I make mine separately, because Adam makes it too sweet.
Adam: You don’t make it sweet enough.
Zena: Yuck! Don’t listen to him. Here’s the right way to make it: put four teaspoons of pink lemonade powder into a glass, like this, and then fill the glass with water. Perfect!
Olivia: Okay. How many teaspoons of powder do you use per glass, Adam?
Adam: Oh, I don’t measure, exactly. I just eyeball it. I put water into the glass, like this… Then I shake some powder out of the package, like this…and stir it a bit. Look at the color. It’s about as pink as Zena’s, which means it’s not as sweet as I like it.
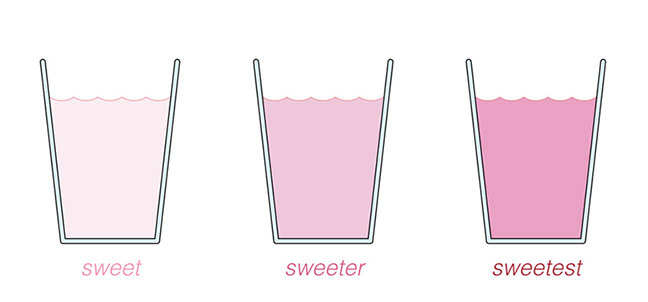
Olivia: That’s kind of interesting: You can “see” how sweet it is by looking at the color.
Adam: Right. The concentration of sugar and of food coloring in the lemonade go together. If you add more powder, the sugar concentration and the pink food coloring concentration both go up. So I add a bit more powder and stir again. There! It’s a nice dark pink. That should be good… Yup! Tastes about right.
Zena: Suit yourself, Adam. Olivia, let’s get you a drink.
Olivia: Thanks. I’m not sure how much of the powder to use. I guess I’ll try four teaspoons, like Zena. It’ll be easier to add more powder and make it sweeter than to take it out once it’s mixed in. (Olivia makes herself some lemonade and takes a sip.) Hmm. Actually, even that is a little too sweet for me.
Zena: If you want it less sweet, you can just dilute it with a little more water.
Olivia: “Dilute”?
Zena: Yeah, dilute: Make it less concentrated.
Adam: Sure. You’ll have the same amount of powder mix dissolved in your glass, but when you dilute it with more water it’ll be less concentrated.
Olivia: There! It’s not as pink now…and it’s not as sweet. Good.
Zena: Hey, do you think that when we mix the pink lemonade powder in with the water, we get a chemical reaction, like we were learning about last week in Ms. Quintanilla’s class? I mean, we’re making a new substance, right?
Olivia: What new substance? Pink lemonade? That’s just water with sugar, flavoring, and coloring mixed in, isn’t it? I don’t think we’re making a chemical reaction and producing some kind of pink lemonade molecules…are we?
Zena: Why not? There’s obviously some kind of change, and we learned in class that chemical reactions change matter. Maybe water reacts with the powder and makes a new compound.
Adam: Well, wait a minute. I don’t think pink lemonade can be a compound. Look at our three glasses. Mine is the darkest pink and sweetest. Olivia’s is the palest pink and the least sweet. And Zena’s is in between. We learned that a compound combines different elements in definite proportions: all water has two atoms of hydrogen per one atom of oxygen, and so on. But we’ve got three samples of pink lemonade and each one combines its ingredients in different proportions. So if pink lemonade doesn’t have definite proportions, I don’t think it can be a compound.
Olivia: Yeah, I think it’s just water with powder dissolved in it.
Zena: So is “dissolving” not a kind of chemical reaction? It’s definitely a change. Is dissolving a kind of change that doesn’t involve a chemical reaction?
Adam: I think it could be. We did learn that not all changes in matter are chemical reactions. Remember: when a nail rusts, that’s a chemical reaction, but when it just bends, that’s a physical change rather than a chemical reaction. So maybe dissolving is another kind of physical change that doesn’t involve making new compounds. Maybe dissolving just mixes the molecules of different compounds together.
Olivia: The powder has sugar molecules, and I suppose the coloring and flavoring also come in the form of molecules. Maybe all those molecules just get mixed in with the water molecules. And if the molecules don’t change, there’s no chemical reaction.
Zena: Hmm. Okay, maybe mixing the molecules of the water and the pink lemonade mix is like mixing white rock gravel and black rock gravel. You could mix white and black gravel in different concentrations and get mixtures that would be different shades of gray when you looked at them from a distance. But there wouldn’t actually be any gray rocks; the white and black rocks would all be the same as before. Maybe our glasses of lemonade are like that.
Adam: Well, I’m not sure. But I’ve got a solution. Hey,
Aunt Lucy?
Aunt Lucy: (coming into the kitchen) Hi guys. What’s up?
Adam: We were wondering whether you get a chemical reaction when you stir pink lemonade powder into water. We figured if there was a chemical reaction, there would have to be a new compound, and we weren’t sure whether pink lemonade is a compound, because it isn’t made with consistent proportions of ingredients. Some pink lemonade is diluted, like the weak stuff Zena likes…
Zena: And some is more concentrated, like that sickeningly sweet stuff Adam drinks.
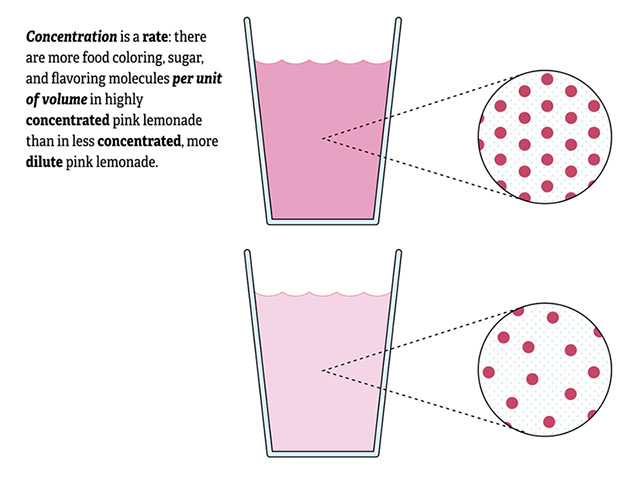
Aunt Lucy: Um, okay. Well, when we talk about concentration, we’re usually talking about a chemical solution, not a compound.
Olivia: A solution like the answer to a problem?
Aunt Lucy: No, I’m using the word solution in a different sense, related to the word “dissolve.” A solution is what you get when you put compounds together and instead of a chemical reaction you get a homogeneous mixture. In a homogeneous mixture, the particles are evenly mixed together, the way the water molecules, sugar molecules, flavor molecules, and coloring molecules are in the pink lemonade. Samples taken from different parts of a homogeneous mixture will be exactly the same.
Adam: So are all mixtures homogeneous?
Olivia: No, I don’t think that would make sense. If we made a smoothie in a blender and you took a sip, you might get a lump of banana. In another sip, you might get more strawberry, or more orange juice, or whatever ingredients you mixed into your smoothie.
Aunt Lucy: Right. A smoothie would be an example of an uneven, lumpy, heterogeneous mixture. Samples taken from different parts of a heterogeneous mixture may have different substances.
Zena: So when stuff dissolves and makes a solution, is that a kind of chemical reaction?
Aunt Lucy: No, dissolving is usually thought of as a physical change, not a chemical reaction. There are other physical changes, too, like when a substance changes from solid to liquid to gas—for example, water changing from ice to liquid water to steam. Those are called phase changes. A substance doesn’t change its chemical composition just because it melts or evaporates. Water is still H2O, whatever phase it’s in.
Olivia: And water is still H2O even when it’s got sugar molecules and other molecules from pink lemonade powder dissolved in it. It’s still water, even though it’s not pure water.
Aunt Lucy: Exactly. In fact, you can divide substances into pure and impure substances. Elements and compounds are pure substances. Another name for pure substances is “chemicals.” Mixtures are impure substances, because they combine two or more pure substances. Within mixtures, you have homogeneous mixtures (also called solutions) and heterogeneous mixtures.
Zena: Wait, slow down. Let me grab some paper. Can you repeat that? We’re supposed to make a poster about chemistry in Ms. Quintanilla’s class for next Tuesday. If we use these categories, maybe we can make a kind of map for categorizing all kinds of matter.
Olivia: I think I’ll do my poster on concentration. I can do the whole thing with pink lemonade!
Aunt Lucy: And I think I’ll have a spoonful of this pink lemonade powder. I like it straight up, with no water!
Adam: Go Aunt Lucy! Now that’s what I’m talkin’ about!
Zena and Olivia: Eeeeeew!
Worksheet: Considering Olivia's poster (20 minutes)
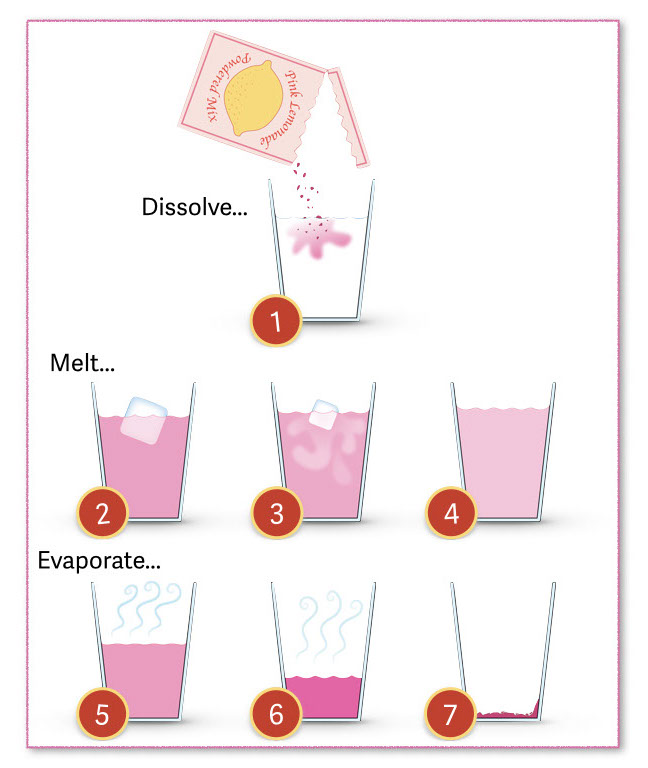
Olivia’s poster illustrates what happens to powder pink lemonade under a couple of different conditions. Read through the introductory text as a class and have students work in partners to answer the questions.
:
As the ice melts into the solution…
- What happens to the volume of the solution?
- The volume increases.
- What happens to the amount of sugar, flavoring, and coloring molecules in the solution?
- They remain the same.
- What happens to the concentration of the solution?
- The concentration of the solution goes down. (Or: The solution becomes more dilute.)
As the water evaporates from the solution...
- What happens to the volume of the solution?
- The volume decreases.
- What happens to the amount of sugar, flavoring, and coloring molecules in the solution?
- They stay the same.
- What happens to the concentration of the solution?
- The concentration goes up. (Or: The solution becomes more concentrated.)
- At which step are the contents of the glass most concentrated and sweet?
- Step #7
- At which step are the contents of the glass most dilute and unsweet?
- Step #4
About blood alcohol concentration (15 minutes)
Read the explanation on blood alcohol concentration (BAC) and the question. (Slide 1)
:
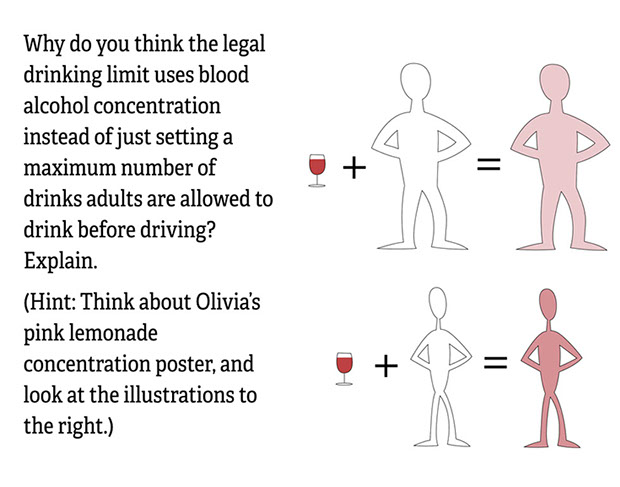
- Why do you think the legal drinking limit uses blood alcohol concentration instead of just setting a maximum number of drinks adults are allowed to drink before driving? Explain.
Before students write an explanation, ask them to discuss the topic with a partner. As students write, encourage them to use precise, academic words and phrases.
:
- Larger people have more blood in their bodies than smaller people. One drink in a small person is going to have a bigger effect than the same drink in a larger person. So to set a maximum number of drinks would not be the best way to tell if a person is getting too much alcohol or not.
:
- A person’s blood alcohol concentration, or BAC, depends on both the size of the person and the amount of alcohol that a person consumes. Since larger people have a greater volume of blood, they will have a lower BAC when drinking the same amount of alcohol as a smaller person. It’s the concentration of alcohol that matters, not simply the total amount.
- We can see the same idea with the medicine that we buy at the drug store. There are usually different dosages for children than adults because of body size and blood volume. Children taking the same amount of medicine as a person twice their size may actually be harmed by the medicine rather than helped.

BETA Version - Please send comments and corrections to info@serpinstitute.org