SciGen Teacher Dashboard
Unit M2
Determining Density
Archimedes & the Case of the Missing Gold
The Basics of Density
Heating Up and Spreading Out
Is Putty or Clay More Dense?
 Interactive: Heating Up and Spreading Out
Interactive: Heating Up and Spreading Out
Duration: Approximately 50 minutes
It's important for students to realize that even though density is a property that describes particular substances such as iron (iron is usually listed as having a density of 7.87 g/cm3) the density of iron and most other substances changes depending on their conditions. This occurrence is most dramatically demonstrated with temperature changes. An interactive set of questions allows students to explore that.
This activity also introduces the important fact that water is used as the reference point for discussing density. Since the density of liquid water is listed a 1 g/cm3, it stands to reason that items with densities < 1 float in water and > 1 sink.
However, it’s important for students to consider that floating happens in substances other than liquid water—in air, for example. In this activity, students compare hot air balloons and helium-filled blimps to begin exploring the fundamental reasons that explain why things float.
LEARNING OBJECTIVE
Students will compare the densities of iron at various temperatures.
Students will learn about the importance of water as a density reference point.
Students will broaden their thinking about floating to include substances other than water.
Materials (one per student or group)
- optional: electronic device (such as computer, laptop, tablet) to use the interactive elements, if not looking at them as a class
Teacher Tune-ups
Teaching Notes
ACTIVITY OVERVIEW
- Pose a question: What happens at a particulate level when iron is heated? (15 minutes)
- Water as a reference point... although water ice is weird! (15 minutes)
- Floating in fluids... including air! (20 minutes)
Pose a question: What happens at a particulate level when iron is heated? (15 minutes)
This part of the lesson plan includes an interactive element that can be used by students in groups or individually. Or teachers can project it to discuss the concepts as a class.
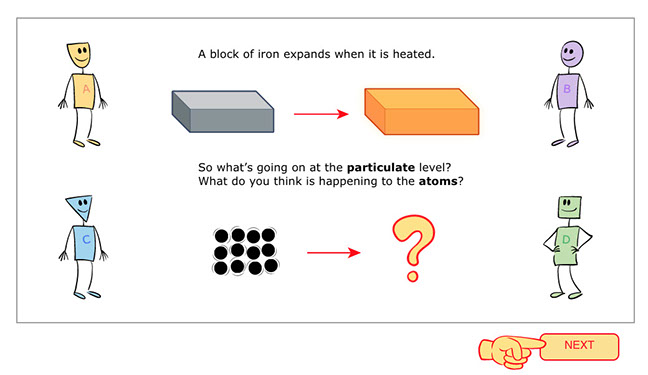
Panel 1: Students are asked to think about a block of iron at the particulate level and share what they think would happen to the iron atoms as they heat up.
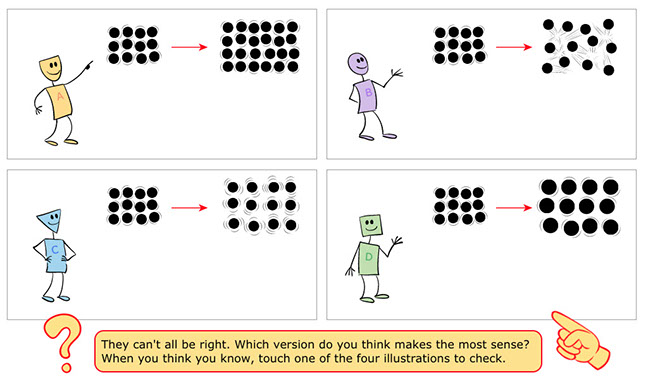
Panel 2: Students examine four illustrations that show four different theories. In a sense, all the slides contain logical conclusions, but three of them are wrong. When students tap on the images they get full explanations.
:
- (wrong) Since things tend to expand when heated, Character A supposes that more iron atoms must be created.
- (sort of wrong) This idea is on the right track, but the increase in temperature would have to be quite dramatic to change a solid block of air to a liquid or gas. The image on Panel 1 shows that the iron is still solid.
- (right!) The important things for students to notice are that iron atoms move faster and bump into each other but as long as the block remains solid, the vibrating atoms stay in the same relative position. Also, the number of atoms does not change, but they do push away from each other so the size of the whole block expands.
- (wrong) This presents the idea that the iron atoms become larger, which is not true.
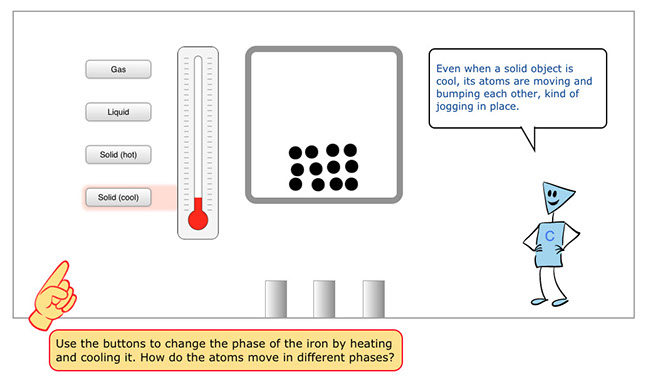
Panel 3: This interactive slide allows students to play with a model that shows atom movement for iron that is in different states: solid (both cool and hot), liquid, and gas.
Water as a reference Point... although water ice is weird! (15 minutes)
Water is very important in discussions of density with students. In general, the density of liquid water is a reference point. Students must learn that the density of liquid water is 1. Reinforce the fact that this designation is intentional—the mass of a cubic centimeter of pure water was determined to be a gram. Water is the standard for measuring density.
This activity is an opportunity for students to explain density and buoyancy in their own words. The key point is that objects that are more dense than water will sink in water, while objects that are less dense than water will float in water. If students are able to articulate the definition of density as the amount of mass per unit volume, or words to that effect, and to express the idea of water and objects pushing each other, so much the better. (If students seem to need an additional prompt, you can ask them whether—and why—a 100 kilogram pine log floats whereas a one gram piece of gold sinks.)
Remind students that what we commonly call water and ice are often called “liquid water” and “solid water” in scientific language.
The density of ice, or solid water, is 0.92 g/cm3.
Floating in fluids... including air! (15 minutes)
A frequent misunderstanding among children and adults is that they think the words fluid and liquid mean the same thing. Actually, gases are fluids too!
One way to help students think about gas as a fluid is to discuss that "floating" is not only about solids in liquids. For example, liquids can float in other liquids (an oil spill is a familiar example), and gases can float in other gases. This fact explains how blimps and hot air balloons can float in the air.
The illustrations show how density is related to floating.
Begin by asking students what they already know about items that float or sink in water. What about their own bodies when they try to swim? Students will know that wood floats and most will have learned that this is because wood is less dense than water.
Remind students that air is also a fluid. Anything that is less dense than air will float. Students will know that helium balloons float—but they may not know that this is because helium is less dense than air.
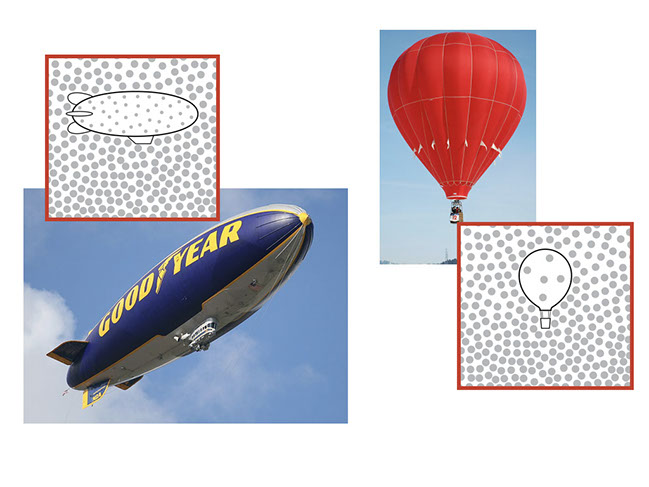
Illustrations:
Helium blimp - Draw students’ attention to the different sizes of the molecules in the air and the helium atoms in the blimp. There are about the same number of air molecules and helium atoms per square centimeter of the illustration (which reflects the fact that a given volume of gas at a certain temperature and pressure will have the same number of molecules regardless of kind). However, the helium atoms are much smaller than most air molecules. Because of this difference in particle size, the helium-filled blimp is less dense than the surrounding air. Therefore the blimp floats.
Hot air balloon - In a hot air balloon, the molecules inside the balloon and in the air are of the same kind, and therefore the same size. When the air in the balloon is heated, the molecules move and spread out. Excess molecules are forced out of the opening at the bottom of the balloon. This expansion due to heating makes the air inside less dense than the surrounding air, so the balloon floats.
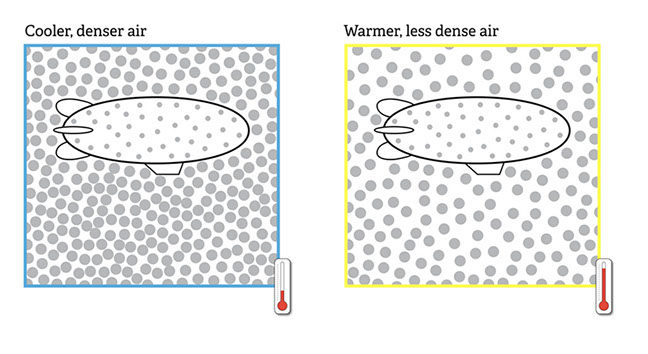
This activity asks students to consider the impact that air temperature has on the speed and ease with which a helium blimp will float.
The particulate view of cooler and warmer air shows that cooler air is more dense. Assuming that the density of the blimp remains consistent in both situations, the greater contrast between its density and that of the cooler air will make it float more easily than it would in the warmer air. Because the blimp is more buoyant in colder air, it can carry more weight.
The following thought experiment may clarify how the contrast between the densities of a floating object and the medium in which it floats affects buoyancy. Students know that a helium balloon floats in air. Ask them to imagine pushing a helium balloon down through the air and then down into water. Assuming that the helium balloon does not burst and is not dramatically compressed (compression would change its density), will it be easier or harder to push the balloon down through water, compared with air? Many students will grasp intuitively that the balloon will be pushed upward more forcefully by the water than by the air. This contrast in “buoyant force” is caused by the different densities of water and air.
BETA Version - Please send comments and corrections to info@serpinstitute.org