SciGen Teacher Dashboard
Unit M1
Small, Smaller, Smallest
 Lesson: Atoms Forming Groups
Lesson: Atoms Forming Groups
Duration: Approximately 45 minutes
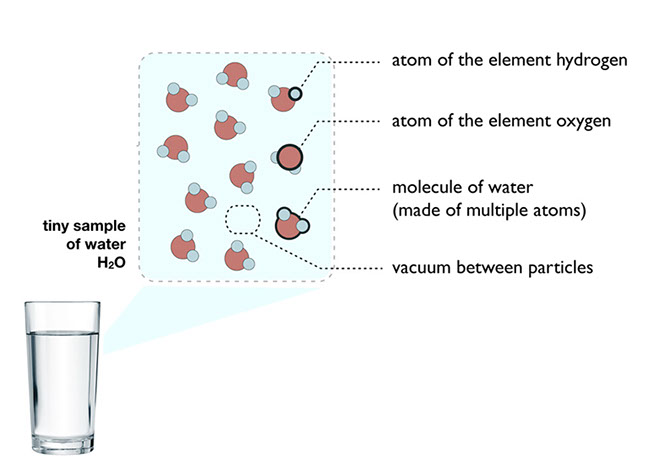
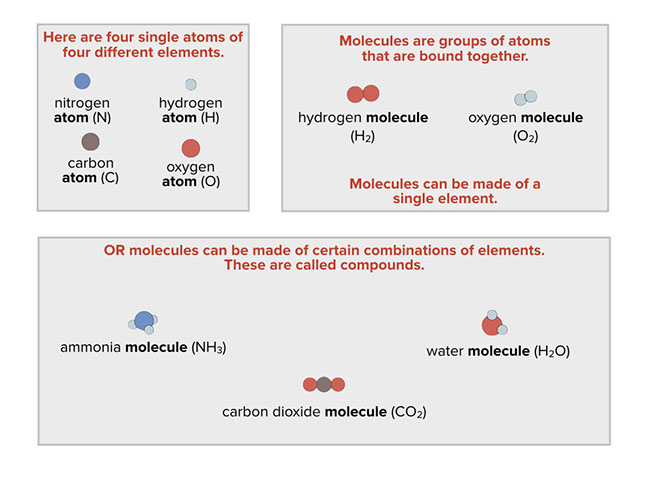
Everything is made of atoms, but not all atoms are identical. In fact, there are over 100 kinds of atoms. About 92 of them occur naturally, and the others have been made by scientists in the laboratory. There are hydrogen atoms, helium atoms, carbon atoms, oxygen atoms, and so on. Substances that are made of just one kind of atom are called elements. Atoms of the same element are all essentially alike, and they’re different from atoms of other elements.
If atoms were only found in their elemental form, there would only be about 100 different substances in the world. But there are millions of different substances. Chemists study substances and how they change through the rearrangement of their atoms. The great variety of substances in the world is possible because the atoms of different elements join together to form compounds. Together, elements and compounds are called chemicals. A chemical does not have to be some fancy substance cooked up in a laboratory. Everything, from the air you breathe to the materials in your body, is made of chemicals.
Some common compounds are water, carbon dioxide (which you exhale with every breath), and ammonia (which is often used in cleaning fluids). Compounds have definite proportions of the elements that go into them. For example, water always has twice as many hydrogen atoms as oxygen atoms; carbon dioxide always has one carbon atom for every two oxygen atoms; and ammonia always has one nitrogen atom for every three hydrogen atoms.
A molecule is a group of two or more atoms linked together in a particle with a clearly defined boundary. Just as atoms are the smallest particles of elements, molecules are the smallest particles of molecular compounds like water, carbon dioxide, and ammonia.
LEARNING OBJECTIVE
Students will learn to differentiate between and apply the terms element, atom, compound, and molecule.
Teacher Tune-ups
Teaching Notes
ACTIVITY OVERVIEW
- Define and discuss terms for particles (15 minutes)
- Turn and Talk: Determine the proportions of three compounds (10 minutes)
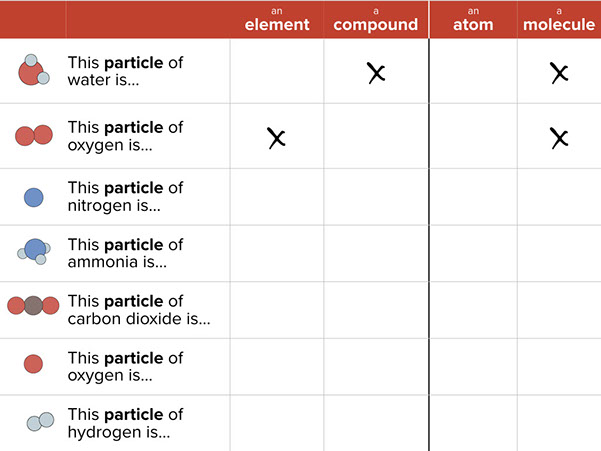
- Partner Work: Complete a table using the terms atom, element, molecule, and compound (20 minutes)
Define and discuss terms for particles (15 minutes)
Turn and Talk: Determine the proportions of three compounds (10 minutes)
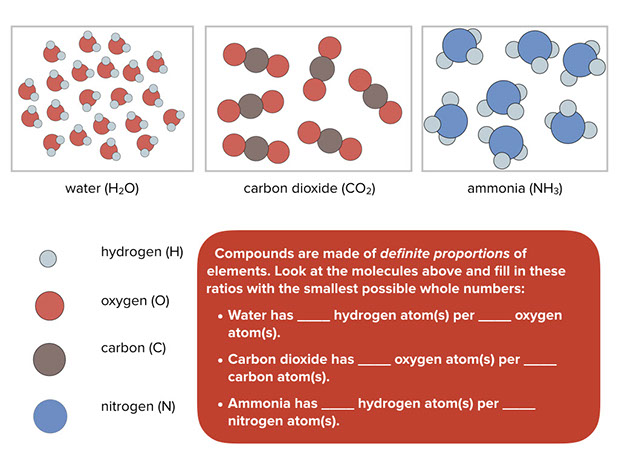
Using the models students can determine the proportions of several common compounds.
:
- Water has 2 hydrogen atoms per 1 oxygen atom.
- Carbon dioxide has 2 oxygen atoms per 1 carbon atom.
- Ammonia has 3 hydrogen atoms per 1 nitrogen atom.
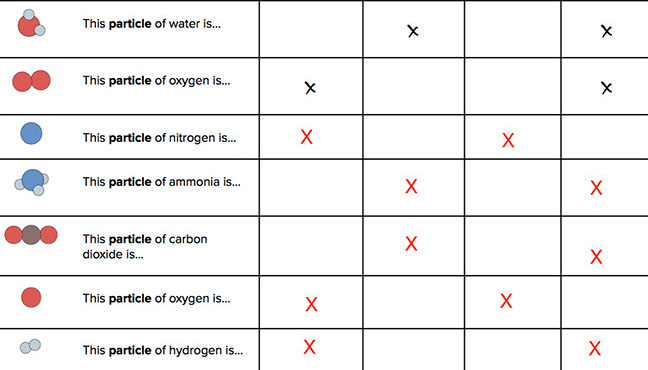
Partner Work: Complete a table using the terms atom, element, molecule, and compound (20 minutes)
:
BETA Version - Please send comments and corrections to info@serpinstitute.org