SciGen Teacher Dashboard
Unit M1
Small, Smaller, Smallest
 Scene: Playing Halfsies
Scene: Playing Halfsies
Duration: Approximately 50 minutes
In this Science Scene, a dialogue to read aloud as a class, students take the roles of three friends discussing what would happen if they could divide a brownie into its smallest parts. The students agree to imagine that the tools were available for them to do so, and begin asking at what point would the tiny parts no longer be a brownie. They rely on an adult to help them conceptualize these small components that comprise matter. She tells them about two different ancient philosophers’ attempts to explain what happens when we divide matter at an incredibly fine scale. After the scene, students examine scientific evidence over the last 200 years and try to determine which of the ancient theories seems closer to being correct.
LEARNING OBJECTIVES
Students consider and compare two ideas about what matter is: a continuous substance or tiny particles.
Students look at evidence discovered over time and consider which theory is supported.
Teacher Tips
- Think ahead about how you will assign the roles to read the script aloud as a class (or, less ideally, in small groups). Note that students often willingly take on roles regardless of the gender of the characters.
- While it is possible for the students to read the PDF of the script online, we suggest printing the script for the students so they can hold it in their hands and mark it as they read. Consider scaffolding the lesson for English language learners: you could let them preview the script before the lesson or read the dialogue silently once and ask questions to a partner.
- Prior to the reading the script, explain to students that they will encounter new vocabulary terms and ideas that may not be familiar. Reassure them that this lack of familiarity is okay. The reading is meant to introduce the ideas, not explain them completely.
- Review the focus words of the week. The focus word chart linked on the unit overview page should be used as a resource for students to review definitions and sample sentences.
Teacher Tune-ups
Teaching Notes
ACTIVITY OVERVIEW
- Set the context (10 minutes)
- Engage with the script (20 minutes)
- Continuous or particles: consider the evidence (20 minutes)
Set the context (10 minutes)
Ask students to turn and talk in order to discuss whether they think that the matter that makes up a nugget of silver, a glass of water, and the air inside of a balloon is made up of tiny particles or is made of a continuous substance. Let students know that by the end of the lesson they will now much more about this.
Consider previewing the cartoon. Ask students to think about which cartoon character is right. Discuss with class.
You might want to stop when Zena suggests that Adam imagine that there is a tool that would allow for them to continue to divide the brownie. Ask the class if they think “using the imagination” in this way is useful when considering scientific ideas.


Engage with the Script (20 minutes)
Choose four students to read the parts of Adam, Zena, Aunt Lucy, and Olivia in the Science Scene. The script is an informal introduction to atomic theory and the nature of matter. The students will be discussing what would happen if they could continue to divide a brownie in half. Would the smallest part still be a brownie, or does it become something else?
Adam: Hey, Aunt Lucy! These are the best brownies ever. Thanks for baking them.
Aunt Lucy: (from another room) You’re welcome. You guys can go ahead and finish them up.
Zena: There are just two left. Olivia, you’re the guest here, so you can have one. Adam, let’s make the other one last a while. Let’s play halfsies.
Olivia: (with a mouthful of brownie) What’s “halfsies”?
Zena: That’s where one of us takes half, and then the other takes half of what’s left, and we go back and forth like that forever, so that we’re never completely done eating the brownie.
Adam: Well, that’s unfair, because whoever goes first gets half of the whole, plus more pieces later. But on top of that, the whole idea is crazy because we would have to finish the brownie sooner or later. Eventually, someone will eat the last crumb.
Zena: I’ll let you go first, so you don’t have to worry about the fairness thing. It’s worth it to me because it’s not ridiculous. Playing halfsies will make the brownie last forever. Forever, I tell you! (She laughs like a mad scientist in a movie. Adam rolls his eyes.) There will always be another half left, no matter how small a half it is. There’s no such thing as a smallest particle of brownie. It might take a magnifying glass, a razor blade, and a steady hand, but we’ll keep cutting those crumbs in half!
Adam: That’s the goofiest idea I’ve ever heard. Obviously at some point, the remaining crumb of brownie would get too small to see, even with a magnifying glass. And at some point, you would get down to a crumb that was smaller than the width of the edge of a razor blade. So you couldn’t cut it.
Zena: (waving her hand dismissively) I don’t care about those practical problems. In fact, you can have the whole brownie for all I care, but only if you admit that I’m right in theory. Just for the sake of argument, imagine that every time we cut the brownie in half, we also got a magnifying glass that was twice as powerful as the one we used before, and a knife that was twice as sharp as the one we used before. With those magical tools, we could keep dividing the brownie in half no matter how small it got, and we’d never be done.
Olivia: Um, if you guys are just going to talk, can I have that last brownie?
Zena and Adam: No!
Adam: Even if we could always use greater magnification and a sharper knife, I think at some point we would come to a smallest piece of brownie.
Zena: Why? Whatever piece you’ve got must have two halves, right? Just like in math, there’s no number so small that you can’t divide it by two.
Adam: I’m saying that once you cut the brownie enough times, you would get to a particle so tiny that if you cut it apart, you wouldn’t have brownie any more. Instead you’d have…I don’t know…maybe tiny pieces of the ingredients that went into the brownie in the first place. You’d be cutting a tiny piece of sugar away from a tiny piece of flour, salt, baking powder, egg, or chocolate. A brownie is only a brownie if it has all those things, so you’d be breaking your teeny-weeny crumb into parts that aren’t brownie substance anymore.
It’s like if you take a parking lot full of cars and keep dividing it in half, you still have cars for a while. But at some point, you get down to one car. After that, if you keep dividing, you end up with some part of a car—a wheel or a bumper or something—but that’s not a car anymore.
Zena: A car is made of separate parts, but I think that’s different from a brownie. A brownie is made of ingredients, but they’re all blended together into a smooth batter, and when you cook the batter, they all sort of dissolve and melt together and puff up in some kind of chemical reaction, right? And it all becomes—ta da!—solid brownie through and through. And then it’s halfsies, baby, forever and ever!
Adam: I bet Aunt Lucy would know about this. She took a lot of chemistry in college for her engineering degree, didn’t she? Hey, Aunt Lucy—
Aunt Lucy: (coming into the room) Hey, yeah, I couldn’t help overhearing your conversation. Actually, you two are repeating a very old argument about the nature of matter. Back in ancient Greece, there was a philosopher named Democritus who believed that everything was made of tiny particles he called atoms. He thought an atom was the smallest piece of something and couldn’t be cut in half.
Olivia: So that’s like Adam’s theory, right?
Aunt Lucy: Right, Adam’s theory of atoms. But not everyone agreed with Democritus. Another philosopher named Aristotle had an especially negative reaction to atomic theory. Aristotle couldn’t imagine absolutely empty space between particles of matter. He wrote that “nature abhors a vacuum,” meaning there is no such thing as space without any matter in it. Since he couldn’t imagine empty space between atoms, he didn’t believe in atoms. He thought matter had to be continuous instead of separated into particles. So for Aristotle, there was no smallest possible piece of a substance.
Olivia: That’s like Zena’s theory that brownies could be divided in half infinitely, if you had a sharp enough knife.
Aunt Lucy: Right. Aristotle thought of matter as being continuous, flowing together without any gaps, like water. Democritus thought of matter as being made of particles. He compared the basic structure of all matter to sand, not water. Democritus thought even water was like sand, if only we could look at it closely enough.
But Aristotle was much more famous and influential than Democritus. So people believed for thousands of years that Aristotle was right about matter being continuous, not particulate. It wasn’t until the start of the nineteenth century that chemists started taking atomic theory seriously again.
Zena: The nineteenth century is the 1800s, right? Why did scientists start believing in atoms then?
Aunt Lucy: Scientists observed various things about the behavior of matter that were easier to explain with atomic theory than without it. The evidence convinced them atoms were real.
Adam: What sort of evidence?
Aunt Lucy: (leaving the room) Why don’t you three look into that?
Olivia: So…Do you guys want to go thirdsies on that last brownie?
Adam and Zena: No!
Continuous or particles: consider the evidence (20 minutes)


(PDF of script includes images to the right)
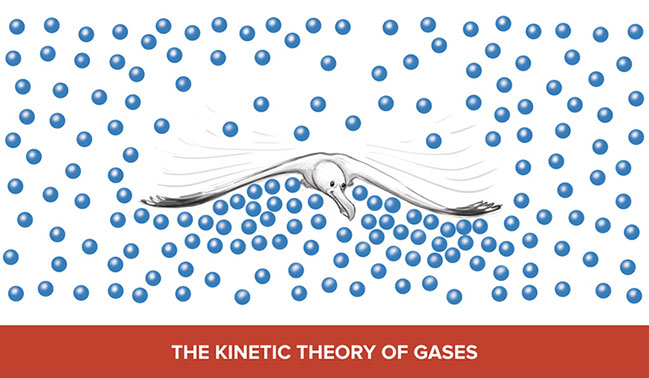
At the end of the scene, Aunt Lucy suggests that the friends conduct a little research to learn more about the evidence supporting the existence of atoms (Democritus’ theory). There are three examples of evidence that support the atomic model: the kinetic theory of gases, Brownian motion, and scanning tunneling microscopy.
Students should read the explanations and examine the illustrations. Ask students to explain how the illustration demonstrates evidence for the atomic model of matter–or Democritus’ theory.
Here are examples of ways that students might explain how the illustrations support the atomic model.
:
- Seagull flying: The seagull’s wingbeat “scoops” atoms closer together underneath creating more density and pressure below the wing than above. This pushes the seagull up.
- Brownian motion: The microscope showed that little parts of pollen were moving around in a random way, suggesting that they must be colliding with something, like bumper cars at an amusement park. However, the things that they are bumping into are so small that they couldn’t be seen even with a microscope.
- Scanning tunneling microscopy (STM): The new technology actually showed that the surface of an object is made of little parts or what scientists called “atoms.” They just never were able to see the parts until the technology made it possible.
BETA Version - Please send comments and corrections to info@serpinstitute.org