SciGen Teacher Dashboard
Unit L2
Environmental Impact
Demo: Killer Crystals
 Duration: 60 minutes plus at least one day to see crystallization
Duration: 60 minutes plus at least one day to see crystallization
After reviewing the results of rising salinity levels on flamingo chicks, students model the process of crystallization that takes place in Lake Natron when freshwater levels are suppressed. Students then use their model to estimate the minimum level of fresh water that is necessary to prevent crystallization.
LEARNING OBJECTIVES
Students simulate the threat to the young chicks of a flamingo population.
Students infer ways that rising salinity could impact a variety of living things.
Students observe crystallization in a model of Lake Natron and use the model to predict the effects of changing levels of salinity in the habitat of the flamingo.
Teacher Tips
- Consider accompanying this demo with at least one of the other flamingo-related lessons in this unit (linked above) to help students understand why crystallization poses a danger to flamingo chicks—while also maintaining a balance of viewpoints on the debate over the soda ash plant and creating more industry and revenue for the local economy (the running theme of the unit).
- If you want to touch on the chemistry of crystallization, you can accompany this demo with the lesson on evaporation (second half of M3.3 Interactive: Condensation and Evaporation).
- Do the demo yourself before trying it with students. The length of time it takes for a crystal to grow will depend largely on the conditions of your classroom. Differences in humidity, temperature, and the level of dissolved minerals in your water all affect how long it will take for you to get results.
- This demo works with Epsom salt (MgSO4, magnesium sulfate), not table salt (NaCl, sodium chloride). Pharmacies sell Epsom salt.
- Consider prepping the pipe cleaners by soaking them in the solution and leaving them to dry so that they are pre-coated with tiny crystals that act as "seeds" for crystal growth.
- The hotter the water used to dissolve the water, the more saturated the solution can get. For safety reasons, you don't want the water to be dangerously hot if students are prepping the solutions. Some teachers furnish the students with supersaturated solution they have prepared ahead of time. To get a supersaturated solution, you can heat the water to increase its solubility OR evaporate water from a more dilute solution.
- Some students may need a language scaffold to share their data.
- Transform this demo to a true "lab" by having students make hypotheses and change the conditions of their experimental setup. See Teaching Notes alongside "Do the demo" for more guidance on this upgrade.
 Materials (one per student or group)
Materials (one per student or group)
- Epsom salt (magnesium sulfate)
- hot water
- heat-resistant container (such as a glass jar or paper coffee cup)
-
small, shallow dish, such as a jar lid or the well on the bottom of a small, clear plastic cup
- a non-disposable option: small dishes, like those used for soy sauce (available at many discount / dollar stores)
- small stir stick
- pipe cleaners / chenille stem or similar to represent baby flamingo feet
- tray (to distribute materials and control spills)
- optional: digital scale (to gather mass change data; can be shared between several different groups)
Safety Checks
- Review with your students all your school's safety protocols when doing labs.
- This demo uses hot liquids. Have cool water or burn ointment on hand if sensitive skin touches water that is too hot.
- Wear goggles.
- Wash hands thoroughly after the demo and after handling the salts or salt solution.
Teacher Tune-ups
Teaching Notes
ACTIVITY OVERVIEW
- Safety review (5 minutes)
- Pre-lab discussion (10 minutes)
- Do the demo (20 minutes) / crystal growth (hours)
- Discuss the results (much later: 10 minutes)
Safety review (5 minutes)
Show the slide, which has prompts for students to responses.
Paraphrase:
You all know a lot about safety already! What kinds of guidelines can we have for this demo and the conditions of the classroom to keep us all safe? Some of the things on this list are dangers, others are protections.
Possible responses:
Try not to spill hot liquids! If hot liquids get on your skin, be sure to run it under cool water. Watch out for where your clothing is in relationship to the containers of hot liquids. If a spill happens, move away from the table so that the hot liquid doesn't drip onto your skin. Keep your hair back. Fold back loose sleeves and remove big bracelets.
Keep the cup of boiling water on a tray so if it is knocked over the spill will be contained.
Immediately apply cold water to any burn.
Wear goggles.
Wash hands thoroughly after the demo and after handling the salts or salt solution.
Follow all class safety rules.
Set the context before the demo (10 minutes)

Lake Natron provides a great example of how humans influence the delicate balance of nature on a time scale that nature can’t respond to. The crystallization that occurs is fascinating to look at, and it is also ecologically meaningful.
Ask students to turn and talk to consider these questions with a partner:
What do you know about the habitat of the flamingo?
What influences their habitat?
What dangers are present? Why are they dangerous and for whom?
Show slide. The photos show a flamingo chick and some full-grown lesser flamingos.
Ask:
Why do you think flamingo chicks are more likely to be threatened by salt crystal buildup on their legs as compared to adult flamingos?
Possible Answers:
Young chicks would drown when they tripped because of the salt rings. Crystallization doesn’t affect adult flamingos, whose legs are long and more widely spaced. The same size of crystal bracelet on a flamingo chick that is life-threatening would be a mere nuisance for an adult flamingo.
Do the demo (20 minutes)
Introduce the demo. Students will need to measure, mix, create a “foot,” and set it up in a stable way that can sit without being disturbed for a long time.
Paraphrase:
We will model what would happen to a small "flamingo foot" if it were to sit in alkaline water for long periods of time.
Lake Natron is an alkaline lake, meaning that it is salty. Not salty like the ocean or a potato chip. The solution we'll use today is made of magnesium sulfate dissolved in water. Be sure to wash your hands at the end of the lab.
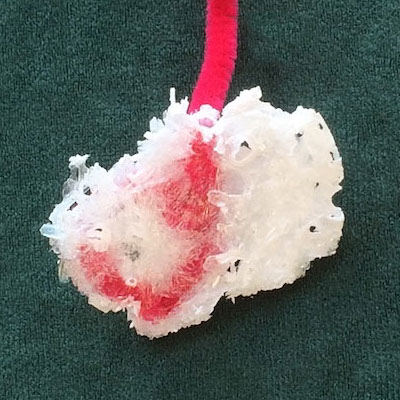
Students follow the procedure described to the right. The setup is quick; waiting for crystals can take hours to days. The rate of evaporation depends on the conditions of your classroom (ambient temperature, exposure to sunlight or heaters, humidity). Crystals only appear when a solution is supersaturated.
If you have access to a cheap digital scale, have students weigh their “feet” before and after the demo. Heavier feet have more crystals.
Because the setups are all the same in this procedure, we have called this lesson a demo not a lab. Upgrade this demo to a true "lab" by having students make hypotheses and change the conditions of their experimental setup. For example: how much water is necessary to prevent the deleterious effects of crystallization? Model the inflow of fresh water by adding variables: students could start with, say, 20mL of supersaturated solution and gather data with different experimental conditions: adding 0–10mL of fresh water per day, for example. Students would determine how much water, as a percentage of the volume of the model lake (20 mL) would counteract the evaporation that leads to supersaturation.
Reflect on or discuss the results (much later: 20 minutes)
- Develop a hypothesis about how to change the outcome, and then write a procedure for an experiment that would test the hypothesis.
- What would happen if you dipped the "flamingo foot" in fresh water periodically? Do you see any connections to the need for fresh water at Lake Natron?
- What other life forms would struggle to survive in an environment where salinity was so high that the dissolved salts crystallized?
- Develop a hypothesis about which animals would find these conditions difficult to survive under, then research to see if any animals of that type live in alkaline lakes like this.
Depending on the conditions of your classroom, crystals will form a day or weeks later. Review what was done in the demo and why before asking students to engage meaningfully with the results.
Ask students to share their data with the class.
Students use these prompts to reflect on their findings.
Address the relationship between the real lake and the model the students have built. Crystals form when the water can’t dissolve any more salt, and the amount of water that is going in the solution decreases due to evaporation. Why does crystallization matter to the flamingos? It doesn’t matter for the adults, but students know it kills the young chicks. Encourage students to take what they have learned so far about environmental impact and try to apply it in new ways.
As adorable as the flamingo chicks are and as heart-wrenching as it might be to consider threats to their habitat, remember to maintain a balance of viewpoints on the debate over the soda ash plant and creating more industry and revenue for the local economy (the running theme of the unit).
Note: Instead of asking students to imagine what would happen in question 3, you can transform the demo into a lab by making salinity a variable students change in the procedure.
Possible responses:
(1) varies
(2) Fresh water would wash off the salts and the crystals will have a harder time growing. Fresh water at Lake Natron would help the flamingo chicks and their ankles stay crystal free.
(3) Fish and aquatic plants are two other kinds of living things that would struggle to survive in an environment where salinity was so high that the dissolved salts crystallized.
(4) varies
BETA Version - Please send comments and corrections to info@serpinstitute.org

