SciGen Unit E3: Delivering Power to the People
Teacher Tune-up
Quick Content Refresher for Busy Professionals
What exactly is electricity?
Electricity results when electrons move around.
Each atom consists of a nucleus (containing over 99.9% of its mass) that attracts a cloud of much-lighter particles called electrons. These electrons arrange themselves in patterns that are specific to each kind of atom (each element, that is). Some electrons either stay attached to their atoms or are shared with another atom to form a molecule. But there may be others that are on the outside and able to move between atoms — such free electrons are the basis of electricity.
Electricity results when some of the electrons are separated from their atoms, creating a surplus of electrons in one place and a deficit of electrons somewhere else. Because electrons are repelled by other electrons, any such imbalance results in forces pushing electrons from the surplus region toward the deficit region. This movement of electrons can be as simple as the flow through a flashlight bulb of the electrons separated in its battery, or as dramatic as a lightning bolt that breaks apart atoms in the air to provide a path that the electrons follow to rebalance the regions of the cloud.
A current is a flow of electrons.
This flow of electrons is called an electric current, and is measured in units called amperes (or amps for short). Each ampere is the flow of 6.24×1018 electrons per second. That’s a lot!
6,240,000,000,000,000,000 electrons
Electrons can move as either direct current (flow in one direction, as when electrons flow from one terminal of a battery to the other through a circuit) or as alternating current (the back-and-forth electron motion that most generators cause—a bit like the back-and-forth motion of a saw). These two types of currents are often referred to as “DC” and “AC,” respectively. Power can be transmitted by either kind of current, and devices exist to convert one kind into the other as needed.
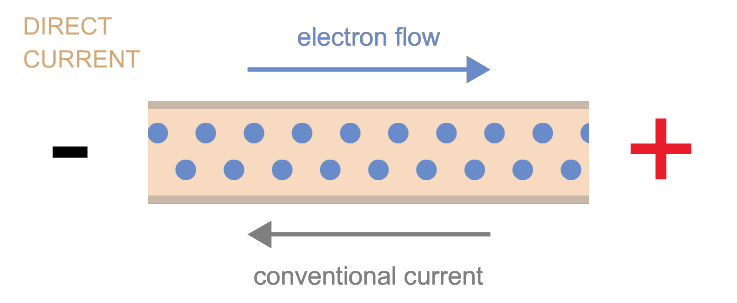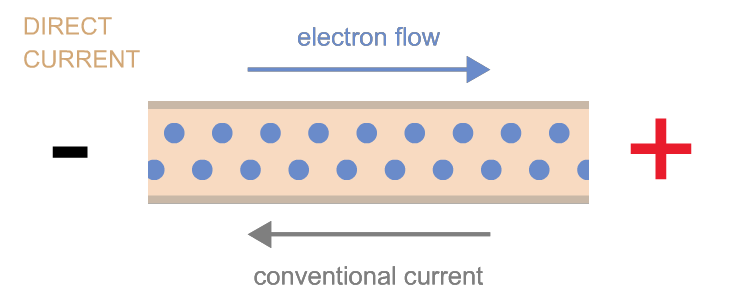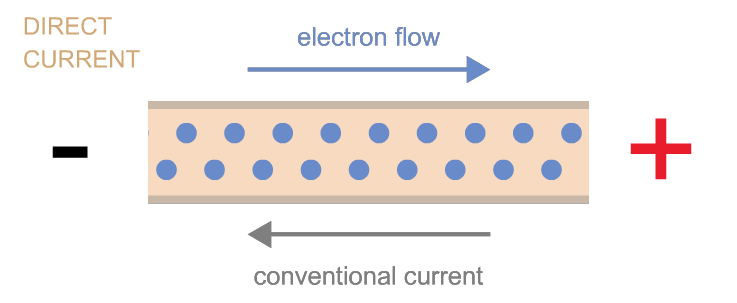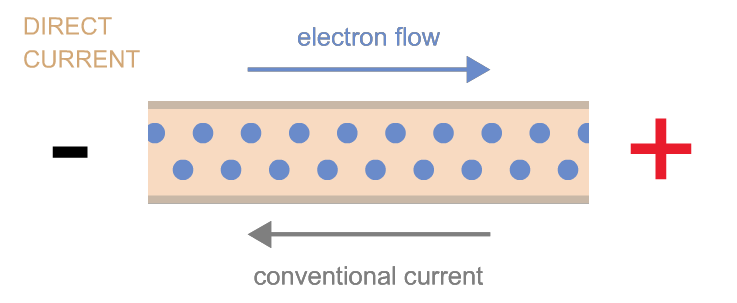
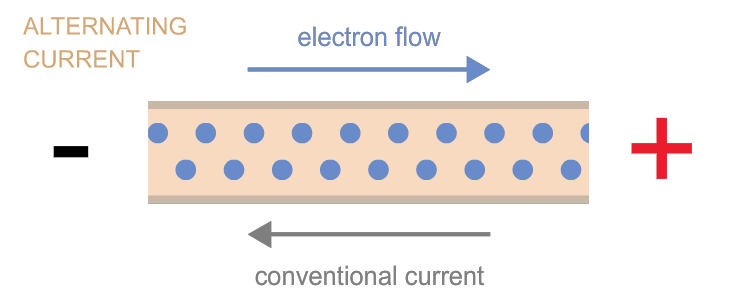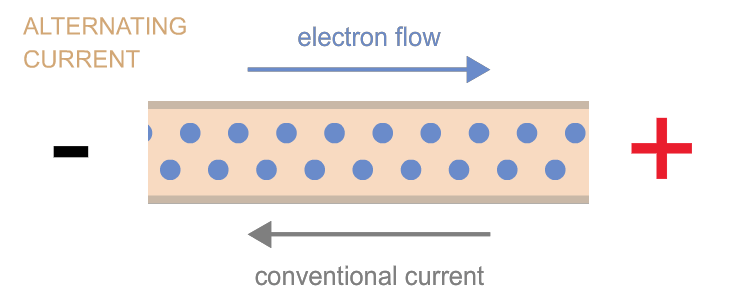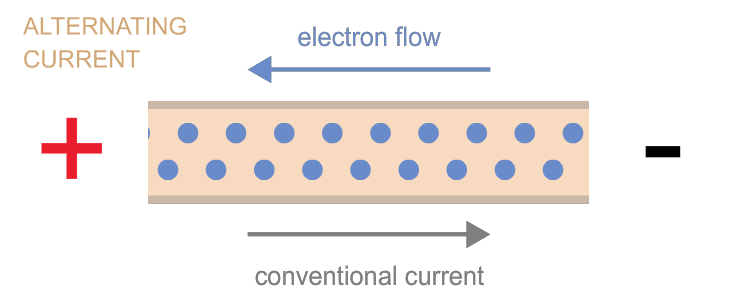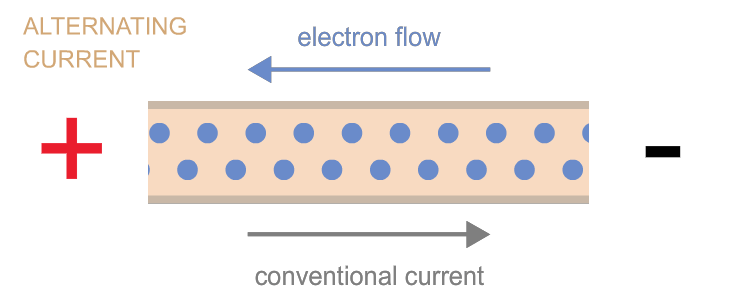
Power is measured in watts.
The power transmitted in electrical circuits is measured in watts, which are joules per second. Common power usages range from 200 milliwatts (i.e., 0.2 watts) when talking on a mobile phone to 4 kilowatts (i.e., 4,000 watts) or more for a home air-conditioning system. Electric-power generators range in size from a few thousand watts for backup power systems to almost a billion watts for generators at large dams and nuclear plants.
Because electrons repel one another, an excess of electrons at one end of a wire will create a pressure that pushes the electrons along the wire. The strength of this electromotive force (EMF) is measured in volts. Direct-current (DC) devices such as flashlights, automobiles, and personal electronics usually operate between 3 and 12 volts. Alternating-current (AC) devices such as household appliances usually operate at either 110 or 220 volts. Thousands of volts are used for long-distance transmission of power in order to minimize losses due to electrical resistance. Transformers help to change the voltage several times along an AC transmission path.
Some materials conduct electricity well, others don’t.
Electrons can move easily through some substances, called conductors. The electrons of some other substances, called insulators, are not free to move. All metals are good conductors, with little resistance to electron flow. Water solutions (including in living things) are conductors but provide more resistance. (Pure water, incidentally, is a very poor conductor—but water is almost never pure in the real world.) Glass, rubber, air, and most plastics are good insulators, and so they can be used to keep an electric current from leaking off the desired path. However, very high voltages can break up the atoms of insulators into particles that will conduct electric currents, which is what happens when lightning occurs.
Electrical resistance comes from collisions between flowing electrons and atoms; it is a kind of fluid friction, similar to the slowing of air as wind blows through the trees in a forest. The energy that resistance takes from the current is converted to heat, which is the vibration of atoms. The electrons that stay with the same atoms do not have friction of this kind, due to the special “quantum” behavior of matter at the atomic scale. At temperatures hundreds of degrees below freezing, the electrical resistance of some substances also becomes zero for similar reasons; such “superconductors” are used to make the very strong electromagnets needed for MRI scans.