Density Dilemma
RETIRED BETA VERSION - For current versions of the SciGen materials, please visit serpmedia.org/scigen
Floating in Fluid
Solids are not the only substances that have density. Water and air have density that changes too. Density plays a key role in determining whether objects will sink or float in fluids and gases.
Gold sinks in water because gold is denser than water, and pine floats in water because pine is less dense than water. In fact, the densities of different substances are often compared to that of liquid water.
density of gold > density of water > density of pine
 TURN AND TALK
TURN AND TALK
- With a partner, figure out how you would explain to a second grader why gold sinks and pine wood floats.
 Water Floating in Water
Water Floating in Water
The density of a substance usually decreases as temperature increases. One familiar exception to this rule is water.
Ice floats in liquid water, so ice must be less dense than liquid water. Ice is actually kind of a strange case. Usually, substances expand and become less dense when they are warmer, and shrink and become more dense when they are colder. Liquid water at room temperature, for example, is slightly more dense than hot water.
When water freezes, it expands slightly and becomes less dense. Why? Well, when water molecules stop sliding past each other and form solid ice, they become organized in a way that actually spreads them apart slightly, so that there are fewer molecules per cubic centimeter. The white space in these illustrations represents empty space:
Liquid Water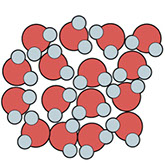 Solid Ice
Solid Ice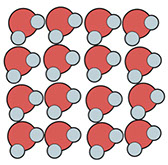
Water is one of the few things we see in three different states of matter on an everyday basis, and it behaves unlike other substances.
Floating in Air
An object placed in a fluid will float if it’s less dense than the fluid, and sink if it’s more dense than the fluid. Remember, a fluid can be either a liquid or a gas. The typical cases we see around us are things that float or sink in air, and things that float or sink in water. Iron is so dense that it sinks in water, whereas helium floats in air because it is less dense than air. Cork is less dense than water but more dense than air, so it floats on water but not in air.
Remember, density is the ratio of mass to volume. So at a particulate level, the density of a substance is determined by two things: how massive the particles of the substance are, and how closely packed or spread out those particles are. These two aspects of density lead to two basic strategies for making lighter-than-air vehicles work.
Helium Blimp
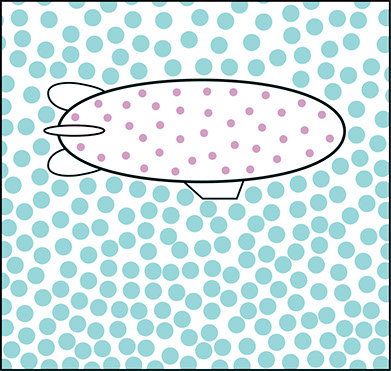
Helium-filled blimps and hot air balloons both float in the air by holding large volumes of low density gases. In a helium blimp, the gas is helium (He). At the same temperature and pressure as the surrounding air, helium has just as many atoms as the surrounding air has molecules. But the helium atoms are much less massive than the air molecules, so the helium is less dense.
Hot Air Balloon
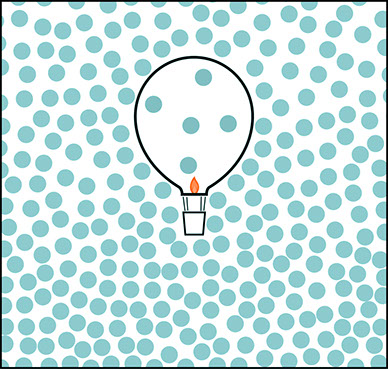
In a hot air balloon, the air inside the balloon has pretty much the same kinds of molecules as the surrounding air. Compared to helium atoms, the nitrogen molecules (N2) and oxygen molecules (O2) that make up most of the air both inside and outside the balloon are pretty massive. But the molecules of heated air move fast and spread out more. Many of them are pushed out of the opening at the bottom of the hot air balloon, leaving the hot air inside the balloon less dense than the surrounding air.
It’s not just what’s inside the blimp or balloon that matters. The density of the air outside changes in different weather conditions. When the air is cold, it is denser because its molecules stay relatively close to each other. When the air heats up, its molecules move faster and spread out so that the air becomes less dense. Compare the two illustrations below. One shows the blimp in cooler, denser air, and the other shows the same blimp in warmer, less dense air.
Blimp in Cooler, Denser Air
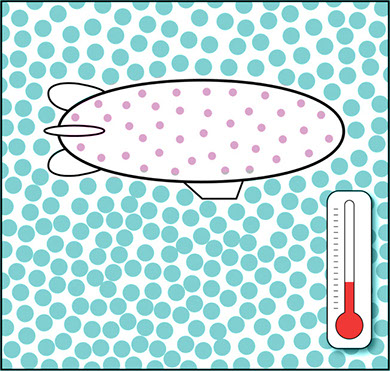
Blimp in Warmer, Less Dense Air
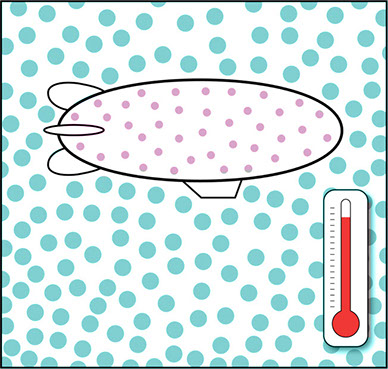
In both cases, the helium in the blimp is less dense than the surrounding air. But the blimp will rise more quickly and float more easily in one case than in the other. The air temperature might affect how much weight the blimp can lift—how many passengers or how much cargo it can carry.
 TURN AND TALK
TURN AND TALK
- Do you think the blimp can carry more weight in cooler air or warmer air? Explain.
-
 Both helium blimps and hot air balloons are airships navigating the air. How do submarines and boats float in a body of water? Neither one uses flames to warm the water, but there are similarities in how these water vehicles become and remain buoyant.
Both helium blimps and hot air balloons are airships navigating the air. How do submarines and boats float in a body of water? Neither one uses flames to warm the water, but there are similarities in how these water vehicles become and remain buoyant.
- How do boats float? Why do they sink?
- How do submarines float? Why do they sink?
- Is a submarine more like a blimp or a hot air balloon?
- Is a boat more like a blimp or a hot air balloon?
- How can you make a hot air balloon lose altitude?
- Some bars of soap sink, but others can float in water. How can a solid object float in liquid? If your floating bar of soap were five times as large, would it still float, or would it sink to the bottom of your bathtub? What if you had a soap bar the size of a dining table, made out of that same solid, floating soap? Would it sink or float? Why?