Density Dilemma
RETIRED BETA VERSION - For current versions of the SciGen materials, please visit serpmedia.org/scigen
IN THE LAB: Density Playground
Density is the ratio of mass to volume. Put another way, density equals mass divided by volume. Density can be measured in grams per cubic centimeter (g/cm3), or in other units of mass and volume.
The density of a substance doesn’t depend on the size of an object made of that substance any more than the color of the substance does. For example, the four blocks of gold below all have different masses, and they all have different volumes. But they all have the same density (not to mention the same color):

mass: 9.65g
volume: 0.5cm3

mass: 19.3g
volume: 1cm3

mass: 38.6g
volume: 2cm3

mass: 154.4g
volume: 8cm3
What is the density of gold? To find out, divide the mass of each block by its volume:
The second one, with no number in front of the cm3, might have seemed either pretty obvious or pretty tricky to you. When you just have a unit like cm3 in an expression like 19.3g/cm3, it means one cm3 (not zero cm3!). This expression shows the “unit rate,” which means it tells you how many grams there are per one cubic centimeter. That’s what we use to describe the density of gold, and you can see by doing the math that it’s the same for all four gold objects. They all have the same ratio of mass to volume.9.65g/0.5cm3 = ______ g/cm3
19.3g/cm3 = ______ g/cm3
38.6g/2cm3 = ______ g/cm3
154.4g/8cm3 = ______ g/cm3
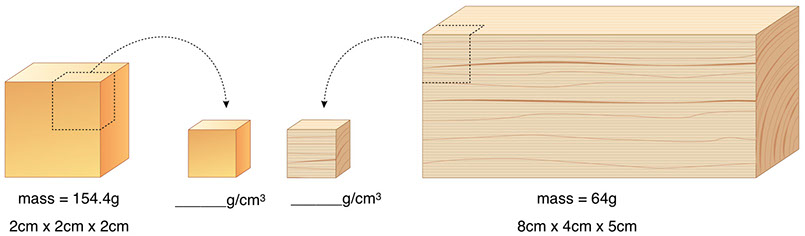
Density helps us compare different substances—not just different objects, but the substances of which objects are made. Consider the block of gold on the left below, with a volume of 8cm3 and a mass of 154.4g, and the block of pine wood on the right, with a volume of 160cm3 and a mass of 64g. Just comparing the mass 154.4g to the mass 64g doesn’t really get at the difference between these two substances, because the particular objects we’re comparing are different sizes. But the density of the two substances tells us something essential about gold and pine wood, something that doesn’t depend on the sizes of the objects. To compare the density of two materials, you consider pieces of each material with the same volume, and compare their masses. To do this without actually chopping pieces out of objects, you divide the mass of each object by its volume.
Find the densities of gold and pine below:
Density is a very basic idea in science, but it can be very confusing sometimes. In this lab, you will work with a partner to come up with some simple demonstrations.
Materials (per partnership):
- Scale that measures down to 0.1g
- White Modeling Clay
- White Modeling Dough
- Ruler
- Graduated Cylinder 100mL
- Jumbo Paper Clip
- Wood Block
- Eraser
- Lab Notebook
- Large Sticky Notes, Easel Pad, or Butcher Paper
- Chart Paper
Part 1. Making Bricks
- Create two clay “bricks” (rectangular prisms) with the dimensions 2cm x 2cm x 5cm. Make one brick with modeling dough and the other with modeling clay. Do not mix the two!
- Measure your bricks frequently with a ruler, and change it to have more or less clay. Use the edge of the ruler to cut or smash the brick into a close-to-perfect rectangular prisms with the dimensions 2cm x 2cm x 5cm.
- Calculate the volume of each of the bricks.
Part 2. Measuring Volume and Mass
You may already have knows the formula for volume (length x width x height.) There is another, very different way to determine volume. It involves water, a paper clip, and a graduated cylinder.
- Fill the graduated cylinder about halfway.
- Read the water level in the graduated cylinder. Note it in your lab notebook.
- Submerge one brick by dunking it completely underwater.
- Read the water level in the graduated cylinder again. Note it in your lab notebook.
- Determine the difference. How much water was displaced?
- Using the scale, measure and record the mass of each brick.
Discussion
Why is a gram a gram? Does it have any connection to cubic centimeters? To milliliters?
Check for Understanding: Wood blocks
Without chopping these blocks up, can we find out how much one cubic centimeter weighs? What kind of wood is it?
In small container of water, test the wood and the two clay bricks. Do any of them float?
- Which is heavier: the wood or the clay?
- Which is denser: the wood or the clay?
Read the Archimedes Comic and then come back for Part 3.
Part 3. Fooling with Density
We’re going to pretend to do something bad, sort of the like the guy who got put in jail in the comic story, Archimedes and the Case of the Missing Gold.
- Make a pure, solid clay/dough object.
- Make another solid object that is not pure (both clay and dough mixed).
- Set up a “shop” to display your objects.
- Visit other shops around the classroom. Using rulers, graduated cylinders, water, and a scale, but without cutting into the objects, find the objects that are not pure.